Updated: Cardiac Injury Markers
By Thomas Reppun MD, Associate DLS Medical Director, October 18, 2001
In early November,Diagnostic Laboratory Services will be changing its test systems on Oahu for the cardiac injury markers, troponin I, CK-MB, and myoglobin. There are several reasons for the change from Dade Behring reagents on the Opus Plus testing platform to Beckman Coulter reagents on the Access testing platform:
First, the new Beckman Coulter/Access system has increased sensitivity and increased specificity. We will be able to identify more of the patients who have myocardial injury while at the same time having fewer false positive results.
Second, the new assays utilize heparinized plasma, thereby, avoiding the false positive results currently caused by fibrin strands in partially clotted serum specimens. With no clotting time required before testing, TAT is shortened.
Third, DLS is installing new automated processing and testing instruments.
Many physicians have been uncomfortable with the increased sensitivity of the Troponin I and the CK-MB by mass assay.However, a consensus document authored by a joint committee of the European Society of Cardiology and the American College of Cardiology suggests that myocardial infarction should be redefined as any amount of myocardial necrosis, as indicated by an elevation of troponin I in the setting of clinical ischemia (Eur Heart J 2000: 21:1502 and J Am Coll Cardiol 2000:36: 959).This consensus outlines the need for a troponin assay with exceptional sensitivity, specificity, and precision at low levels.
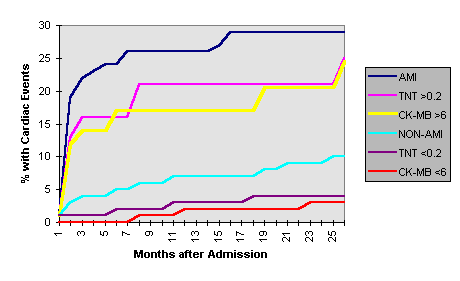
The increased sensitivity to small injuries without EKG evidence of myocardial infarction will require paying closer attention to these patients at high risk of a significant myocardial event within two (2) years. In an article by Dr. Ravkilde et al., using similar normal ranges in unstable angina patients, elevations of either CK-MB or Troponin were clearly associated with an increased risk of cardiac events, repeat AMI and death; the risks approach those of a patient with a classic AMI (see figure below).
In an article by Antman, Troponin I dramatically predicts mortality during the short term, 42 days: Antman EM et al: Cardiac-Specific Troponin I Levels to Predict the Risk of Mortality in Patients with Acute Coronary Syndromes,
The new DLS normal ranges and their expected effects are described below:
Cardiac Troponin I (TnI): Conversion to the Beckman Coulter reagents will have the most extensive effect on the troponin I assay. The new monoclonal assay is directed at portions of the troponin molecule that are not affected by degradation in circulation nor to complexing with cardiac troponin T or C. The improved specificity and use of heparin plasma will result in fewer false positives due to fibrin clots and less interference from heterophile antibodies.
The detection limit for the new assay extends down to 0.01-0.03 ng/ml (in comparison to the current 0.5 ng/ml).
The new upper limit of normal will be set at 0.1 ng/ml (in comparison to 2.0). Patients with a typical/classic acute myocardial infarction will have values > 0.5 ng/ml by the new Beckman Coulter assay ( >0.8 to 1.0 by the current assay, although only flagged as high if greater than 2.0 ng/ml on the current assay).
Approximately 10% of patients who are normal by the current assay will have borderline abnormal TnI by the new assay (0.1-0.5 ng/ml). Borderline results are seen in several situations: 1) early in an evolving MI, 2) late in a resolving MI, and 3) micro-infarcts or myocyte necrosis such as in unstable angina, myocarditis, and CHF.
The sensitivity of the new assay will also result in more frequent detection of late presentation MI and minor myocardial injury in the presence of substantial skeletal muscle injury.
Myoglobin: The normal range for the new assay will continue at 80 ng/ml. However, due to different calibration, approximately 15% more results will be reported abnormal.If paired myoglobin testing is being used to rapidly screen for early evidence of MI by either doubling of the previous result or a change from normal to abnormal, the new assay will have increased sensitivity. However, from comparison studies to date this change correlates well with the new CK-MB and troponin-I assays. Paired myoglobin sampling is usually defined as one sample collected at presentation and a second sample collected 2-3 hours later. Repeat testing after two samples have been shown to be significantly abnormal is unnecessary and should be avoided.
Creatine Kinase MB(CK-MB): The normal range for the new CK-MB (mass) assay will continue to be 5.0 ng/ml, despite the fact that a 5.0 result on the current assay is equivalent to a value of approximately 8.5 on the new assay. The normal range of 5.0 will be reassessed in 2-3 months due to the fact that 95% of normal patients according to Beckman Coulter have CK-MB results < 4.0. According to our comparison studies the new assay will have approximately 15% more abnormals which occur in a group of patients who have abnormal troponin I values by either our old or the new assay. This group of patient results will be more consistent with an acute MI presentation rather than a late MI.
| Current Assay (Dade Behring/Opus Plus) | New Assay (Beckman Coulter/Access) | |
|---|---|---|
| Myoglobin | Normal < 82 ng/ml | Equivalent Value to current 82 = 100ng/ml Normal <80 ng/ml (pending assessment of cut-off @ 70) (approximately 15% more abnormals*) |
| CK-MB | Normal < 5.0 ng/ml | Equivalent value to current 5.0 = 8.5 ng/ml Normal < 5.0 ng/ml (pending reassessment of cut-off @ 4.0) (approximately 15% more abnormals* with existing abnormal troponin value) |
| Troponin-I | Detection Limit = 0.5 ng/ml Abnormal > 2.0 ng/ml Borderline – Not detected Not flagged abn = 0.8 – 2.0 ng/ml Flagged abn > 2.0 ng/ml | Detection Limit = 0.01 – 0.03 ng/ml Abnormal ≥ 0.1 ng/ml Borderline > 0.1 – 0.5 ng/ml (increased abnormals* ~ 7-10%) Traditional/Classic AMI > 0.5 ng/ml (increased abnormals* ~ 10%) Equivalent to current 2.0 = 1.8 ng/ml |
*Increase % abnormal are not cumulative due to overlapping.
The attached spreadsheet contains selected old and new cases for comparison.
If you have any questions, concerns, or advice you may reach me or another pathologist at the Queen’s Pathology Department 808-547-4271 or via DLS Client Services 808-589-5101.